CLAIM: Zimbabwe is the first to introduce injectable long lasting ARV treatment.
SOURCE: Social media account
VERDICT: False
Two years after protests against ViiV in 2022 for not making inexpensive versions of CAB-LA, a PrEP (pre-exposure prophylaxis) injectable, also known as long-acting cabotegravir, available in Africa, the drug is here.
There are various claims of different countries having been the first to roll out the drug to its citizens.
Was it Zambia? Or Zimbabwe? How about Malawi? Or maybe Botswana or South Africa.
One account on X (formerly Twitter) said, ‘Correct me if I am wrong, Zimbabwe is the first to introduce injectable long lasting ARV treatment. Zambia is introducing long lasting Prep treatment to prevent HIV infection’.
Does it even matter? Well, it did enough for a reader to ask FactCheckZW to check the facts.
What is long-acting Cabotegravir (CAB-LA)?
CAB-LA is a long-acting antiretroviral (ARV) medicine patented and produced by ViiV Healthcare, an offshoot of pharmaceutical corporations Pfizer, GlaxoSmithKline and Shionogi.
Delivered as an injection every two months, clinical trials have shown CAB-LA to currently be the most effective form of PrEP. As CAB-LA is more long-lasting and discreet than oral PrEP, and so may facilitate better adherence, it can help turn the tide against new HIV infections globally.
CAB-LA was approved by the US Food and Drug Administration (FDA) in December 2021 and recommended for HIV prevention by the World Health Organization (WHO) in July 2022.
Confusion over rollout
The lack of certainty over where and when this drug was first rolled out arises from the clinical trials.
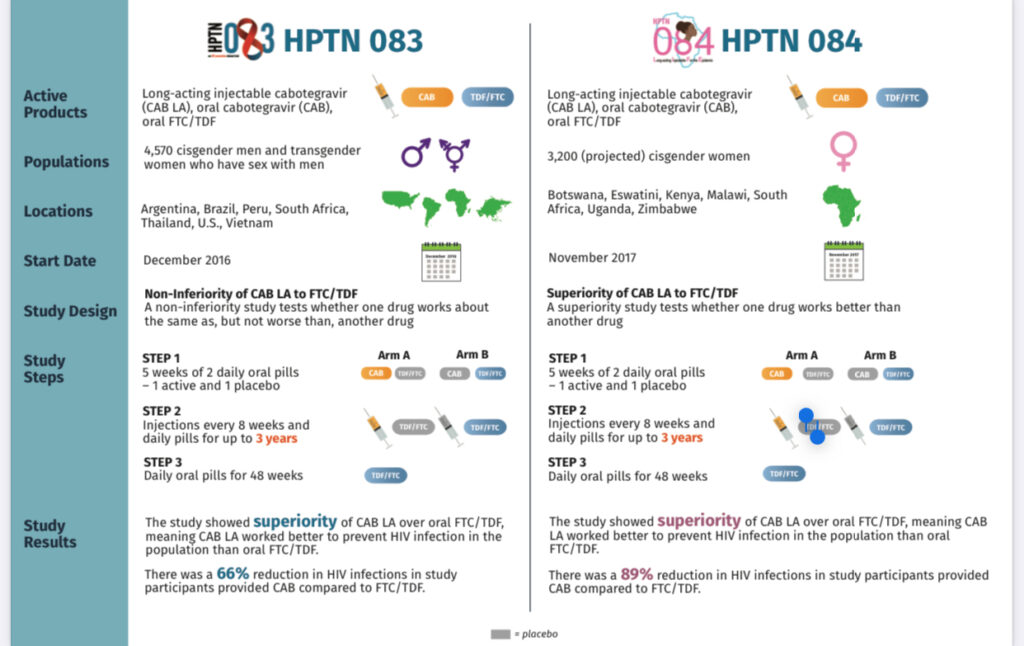
There have been 2 key clinical studies that examined the safety and efficacy of CAB-LA for the prevention of HIV infection: HIV Prevention Trial Network (HPTN) 083 and HPTN 084.
HPTN 083 and HPTN 084 were both randomised, double-blind double-dummy, active-controlled trials comparing the efficacy of CAB-LA and daily oral TDF-FTC in individuals with high risk for HIV infection (one person was worried Zambians were being used as guinea pigs).
HPTN 083 enrolled cisgender MSM and transgender women who have sex with men, and HPTN 084 included individuals assigned female at birth from 7 countries in sub-Saharan Africa with high HIV prevalence. In both studies, participants in the CAB-LA group received intramuscular gluteal injections of 600 mg CAB-LA every 8 weeks, after 2 initial injections given 4 weeks apart.
The African countries involved in the clinical studies were South Africa, Botswana, Uganda, Malawi, Eswatini, Kenya and Zimbabwe.
This means that all these countries got the injection and administered it all as part of the clinical trials.
Zambia, however, is the first country in Africa to have the drug OUTSIDE a study. This followed a donation from the United States government on 9 February 2024, delivered to Zambia as the first shipment of injectable pre-exposure prophylaxis (PrEP) medicine for HIV prevention, making Zambia the second country in the world (after the United States) to offer injectable PrEP outside of a study setting.
Zimbabwe’s Roll Out
Zimbabwe was part of the HPTN 084 study. It has now started rolling out the injectable PrEP at
demonstration sites under a study for Catalysing Access to New Prevention Products to stop HIV (CATALYST).
The study has introduced the dapivirine ring and oral PrEP as HIV prevention choices for women and CAB-LA becomes the third product to be made available.
CATALYST is Maximizing Options to Advance Informed Choice for HIV Prevention (MOSAIC)’s flagship product introduction study.
MOSAIC is a five-year global project funded by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Agency for International Development (USAID) whose primary goal is to help adolescent girls, young women, and other women prevent HIV by accelerating the introduction and scale-up of new and emerging biomedical prevention products.
Zimbabwe’s first injection of CAB-LA (under CATALYST) was administered on 10 April this year in Chitungwiza, Harare.
Eligible individuals who can access the PrEP are serodiscordant couples (where one partner is HIV negative), adolescent girls and young women, and pregnant and lactating women in relationships with men whose status is unknown.
It is also available for people who use and inject drugs, as well as sex workers, high-risk men and the transgender community, among others.
Although Zimbabwe was not the first to introduce CAB-LA on the continent, it was the first country in Africa to approve its use. In 2021, the Medicines Control Authority of Zimbabwe (MCAZ) approved the use of the dapivirine ring and in 2022, it approved CAB-LA, making Zimbabwe the first country in Sub-Saharan Africa to approve the product.
CAB-LA demonstration sites in Zimbabwe are SHAZ! Hub at CitiMed Hospital in Chitungwiza; Ngundu Rural Health Centre; Runyararo Clinic in Masvingo; Plumtree District Hospital; Beitbridge Wellness Centre, and Cowdray Park Clinic, Bulawayo.
Conclusion
Zimbabwe was not the first country in Africa to introduce the long lasting injectable PrEP drug, cabotegravir (CAB-LA). Though it did use it before Zambia, this was as part of a clinical trial that included Botswana, Malawi, South Africa, Eswatini, Kenya and Uganda. Zambia is the first African country to use the drug outside a study. Contrary to the statement, ‘… Zimbabwe is the first to introduce injectable long lasting ARV treatment. Zambia is introducing long lasting Prep treatment to prevent HIV infection’, it is the same drug in both countries. CAB-LA is being used as a PrEP drug in both Zambia and Zimbabwe..
However, Zimbabwe was, indeed, the first country to approve its use on the continent.