CLAIM: Urgent- if you have #YazPlus at home, take it back to the pharmacy with this letter. It’s been recalled due to concern about whether it’s effective as contraception. #consumerprotection
SOURCE: Social media posts
VERDICT: True
Social media has been abuzz with claims of a defective batch of contraceptive pills that has been distributed. The claims here, here and here; are some of those advising those that have bought any from this batch to return the pills: ‘Urgent- if you have #YazPlus at home, take it back to the pharmacy with this letter. It’s been recalled due to concern about whether it’s effective as contraception. #consumerprotection’.
The claims are all true:
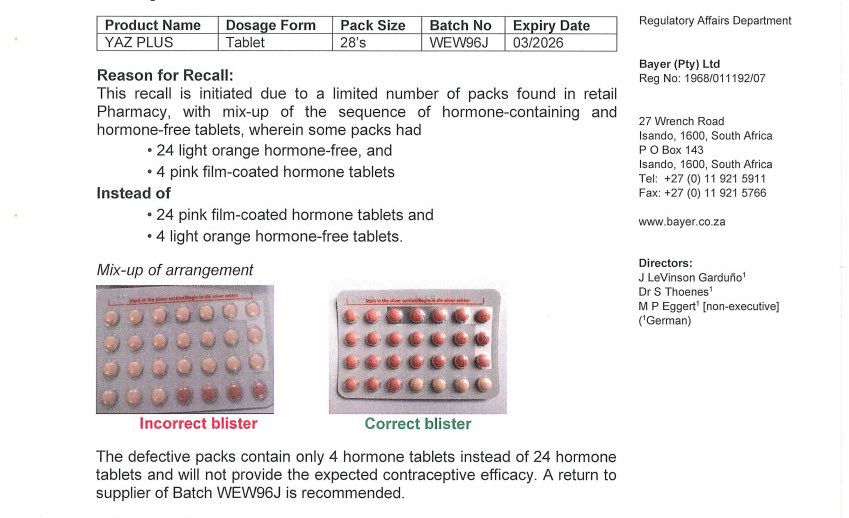
- Batch number: WEW96J expiring in March 2026 is defective
- The placebo and hormone containing pills have been switched
- Consumers need to return these to their nearest pharmacy.
The product recall was first announced by the South African Health Products Regulatory Authority (SAHPRA.) This batch of Yaz Plus birth control pills has the placebo “sugar” pills and hormone-containing pills switched. Instead of the usual 24 hormone tablets and 4 placebos, some packages have been found to contain 24 placebos and only 4 hormone pills.
Although this is a South African product, the Medicines Control Authority of Zimbabwe (MCAZ) has also issued a product recall for the same to alert Zimbabwean consumers who might have purchased the contraceptive.
In a statement dated 22 November 2024 and circulated online, the MCAZ, confirms the recall of the contraceptives in question, adding non-compliance with product quality specifications may result in loss of efficacy, causing potential harm to patients.
“This recall is a precautionary measure to protect the public from the possible inefficacy of the affected batch,” reads the MCAZ statement.
“MCAZ advises all licensed wholesalers, pharmacies, public and private clinics, and hospitals to quarantine any affected units of the product and to cooperate fully with Bayer (Pty) Ltd and the local distributors of the product in Zimbabwe during the recall process.”
The regulator went on to urge members of the public using this contraceptive to check the batch number on their tablet packs, adding if they have the affected batch, they should immediately stop using the tablets, return the affected packs to their pharmacists, and consult their healthcare professionals.
To help members of the public differentiate the correct from the incorrect tablet packaging, the regulatory authorities shared pictures of both:
How do the YAZ Plus contraceptive tablets work?
YAZ Plus contraceptive tablets work by preventing ovulation (the release of an egg from an ovary) and by causing changes in the mucus of the cervix (making it difficult for sperm to penetrate the uterus) and in the endometrium (making it difficult for an egg to implant).
According to the drug manufacturer, Bayer (Pty) Ltd, YAZ Plus tablets are administered as follows:
Dosage and administration
- Tablets must be taken in the order directed on the package every day at about the same time.
- The patient may begin using YAZ PLUS (drospirenone/ethinyl estradiol/levomefolate calcium tablets and levomefolate calcium tablets) on day 1 of her menstrual cycle (i.e. the first day of menstrual flow) or on the first Sunday after her period begins.
- If the patient’s period begins on Sunday, she should start that same day.
- If YAZ PLUS tablets are taken later than day 1 when first starting medication, an additional (barrier) method of birth control is recommended for the first 7 days of use.
- One hormone-containing pink tablet is to be taken daily for 24 consecutive days, followed by 1 hormone-free (folate-containing) light-orange tablet daily for 4 consecutive days. Withdrawal bleeding usually occurs within 2 to 3 days following administration of the last hormone-containing pink tablet (i.e. while the patient is taking the hormone-free (folate-containing) light-orange tablets).
- The patient begins each subsequent course of YAZ PLUS tablets on the same day of the week that she began her first course.
- She begins taking her next course immediately after completion of the last course, regardless of whether or not withdrawal bleeding is still in progress.
Management of missed tablets:
- The risk of pregnancy increases with each hormone-containing pink tablet missed.
- To correct that a woman should match the number of tablets missed with the appropriate starting time for her dosing regimen and a chart can be followed if she misses one or more of her birth control pills.
Conclusion
The claims that there is a product recall of a defective batch of the YAZ plus contraceptive pill are true. Both South African and Zimbabwean health regulatory associations have released statements to that effect. This batch of Yaz Plus birth control pills has the placebo “sugar” pills and hormone-containing pills switched. Instead of the usual 24 hormone tablets and 4 placebos, some packages have been found to contain 24 placebos and only 4 hormone pills.